Curriculum
ISO 17025 2017 General Requirements for the Competence of Testing and Calibration Laboratories - Internal Auditor & Quality Manager Course
Awareness training on ISO 17025 2017 standards
0/7Implementation training on ISO 17025 standards
0/15Internal auditor training on ISO 17025 standards
0/5Evaluation - ISO 17025 internal auditor training
0/3Introduction to ISO 17025 2017 standards
ISO/IEC 17025 enables laboratories to demonstrate that they operate competently and generate valid results, thereby promoting confidence in their work both nationally and around the world. It also helps facilitate cooperation between laboratories and other bodies by generating wider acceptance of results between countries. Test reports and certificates can be accepted from one country to another without the need for further testing, which, in turn, improves international trade. ISO/IEC 17025:2017 specifies the general requirements for the competence, impartiality and consistent operation of laboratories. ISO/IEC 17025:2017 is applicable to all organizations performing laboratory activities, regardless of the number of personnel. Laboratory customers, regulatory authorities, organizations and schemes using peer-assessment, accreditation bodies, and others use ISO/IEC 17025:2017 in confirming or recognizing the competence of laboratories. ISO 17025 2017 standard describes the requirements under the following clauses and the laboratory shall ensure compliance to these requirements.
- Scope
- Normative references
- Terms and definitions
- General requirements
- Impartiality
- Confidentiality
- Structural requirements.
- Resource requirements
- General
- Personnel
- Facilities and environmental conditions
- Equipment
- Metrological traceability
- Externally provided products and services
- Process requirements.
- Review of requests, tenders and contracts.
- Selection, verification and validation of methods
- Selection and verification of methods
- Validation of methods
- Sampling
- Handling of test or calibration items
- Technical records
- Evaluation of measurement uncertainty
- Ensuring the validity of results
- Reporting of results
- Complaints
- Nonconforming work
- Control of data and information management
- Management system requirements
- Management system documentation
- Control of management system documents
- Control of records
- Actions to address risks and opportunities
- Improvement
- Corrective action
- Internal audits
- Management reviews
Benefits of implementing ISO 17025 2017 standards
Major benefits of implementing ISO 17025 2017 standards includes but not limited to:
- Good governance & leadership
- Increase customer satisfaction
- Increase employee morale
- Standout among others
- Control issues before they occur
- Eliminate defects & waste
- Improve supplier performance
- Continual Improvement
- Increase profitability
Laboratory accreditation International Laboratory Accreditation Corporation
International Laboratory Accreditation Corporation for all accreditation bodies globally so that the certificate issued by an accreditation body will be mutually recognized by all other member bodies. ILAC is the international organisation for accreditation bodies operating in accordance with ISO/IEC 17011 and involved in the accreditation of conformity assessment bodies including calibration laboratories (using ISO/IEC 17025), testing laboratories (using ISO/IEC 17025), medical testing laboratories (using ISO 15189), inspection bodies (using ISO/IEC 17020), proficiency testing providers (using ISO/IEC 17043) and reference material producers (using ISO 17034).
Accreditation body
Laboratory accreditation is issued to an organisation by an accreditation body after auditing its management system against the requirements of relevant standard. Most of the countries have a national body who provides laboratory accreditation. Accreditation is the independent evaluation of conformity assessment bodies against recognised standards to carry out specific activities to ensure their impartiality and competence. Through the application of national and international standards, government, procurers and consumers can have confidence in the calibration and test results, inspection reports and certifications provided. Accreditation bodies are established in many economies with the primary purpose of ensuring that conformity assessment bodies are subject to oversight by an authoritative body. Accreditation bodies, that have been peer evaluated as competent, sign regional and international arrangements to demonstrate their competence. These accreditation bodies then assess and accredit conformity assessment bodies to the relevant standards. The arrangements support the provision of local or national services, such as providing safe food and clean drinking water, providing energy, delivering health and social care or maintaining an unpolluted environment. In addition, the arrangements enhance the acceptance of products and services across national borders, thereby creating a framework to support international trade through the removal of technical barriers. The international arrangements are managed by ILAC in the fields of calibration, testing, medical testing, inspection, proficiency testing providers and reference material producers accreditation and IAF in the fields of management systems, products, services, personnel and other similar programmes of conformity assessment. Both organisations, ILAC and IAF, work together and coordinate their efforts to enhance the accreditation and the conformity assessment worldwide. The regional arrangements are managed by the recognised regional co-operation bodies that work in harmony with ILAC and IAF. The recognised regional co-operations are also represented on the ILAC and IAF Executive Committees. ILAC works closely with the regional co-operation bodies involved in accreditation, notably EA in Europe, APAC in the Asia-Pacific, IAAC in the Americas, AFRAC in Africa, SADCA in Southern Africa, and ARAC in the Arab region. 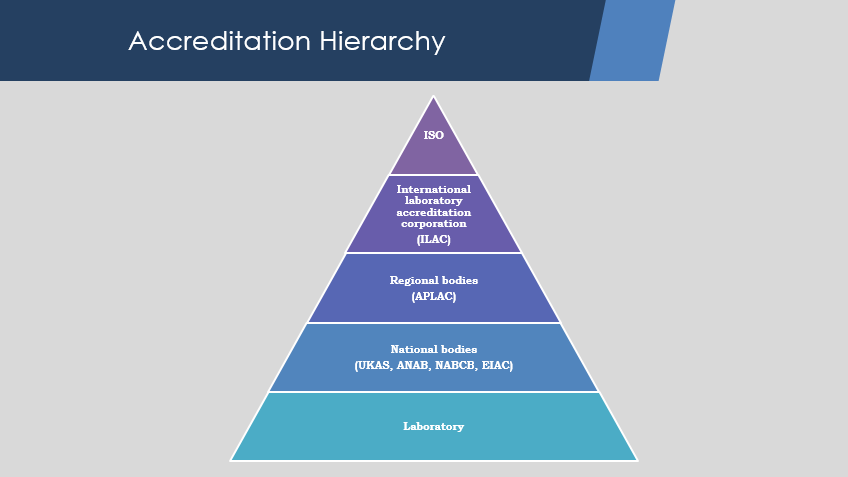 ILAC-MRA Mark
ILAC-MRA Mark
All ILAC Full Members (MRA signatories) are able to enter into an agreement with ILAC to use the ILAC MRA Mark in combination with their own accreditation body Symbol (otherwise known as a Combined MRA Mark). The accredited laboratories and inspection bodies of ILAC Full Members that have signed an agreement with ILAC for the use of the ILAC MRA Mark are able to use the Accredited CAB Combined ILAC MRA Mark. This allows these facilities to provide an instantly recognizable link to the ILAC MRA on their reports and certificates containing the results of calibration, testing and inspections carried out under the scope of their accreditation. Use of the Combined MRA Mark (used by accreditation bodies) and the Accredited CAB Combined MRA Mark (used by laboratories and inspection bodies) is however not mandatory and therefore laboratory and inspection reports and certificates, from accredited laboratories and inspection bodies, may be seen with or without the Accredited CAB Combined MRA Mark. Accreditation Bodies using the Combined MRA Mark, receive the benefits of being able to readily promote their international recognition status, and of being able to provide the same opportunity to their accredited laboratories and inspection bodies. Accredited laboratories and inspection bodies, using the Accredited CAB Combined MRA Mark on their test/calibration and inspection reports, are in turn able to receive the benefits of promoting their accreditation as being internationally recognised.